Introduction
A new antipsychotic drug called KarXT (Cobenfy) was recently approved by the FDA. In the U.S., it will be launched in October 2024.
In this article, I will summarize what I have written about KarXT (cobenfy) so far. I will summarize what I think is particularly important.
Five Advantages of Cobenfy
First, I will write about five advantages of taking Cobenfy.
Advantage 1: High efficacy
Cobenfy is likely to be highly effective.
Its effectiveness for positive symptoms is likely to be very high. At the very least, it is likely to be higher than Risperdal or Olanzapine. Maybe even higher than Amisulpride or Clozapine.
However, we do not know its effect on negative symptoms. In clinical trials, the effect on negative symptoms is not consistent. It may be higher or lower.

Cobenfy’s effect on positive symptoms may be quite high. Its effect on negative symptoms is unknown.
Advantage 2: Effect on cognitive dysfunction
Xanomeline, the active ingredient of Cobenfy, was originally developed as a drug for Alzheimer’s disease. Therefore, Cobenfy may also be effective for cognitive dysfunction in schizophrenia.
In 2008, a pilot study showed the most robust improvement in verbal learning and short-term memory function.
Several tests reached the level of significance, including list learning, story recall, delayed memory, and digit span tests.
However, there were no significant improvements in tests on attention or speed of information processing.
A post-hoc analysis of the Phase 2 study also confirmed the effect on cognitive dysfunction.
However, patients with less cognitive impairment did not seem to benefit as much.
Cobenfy may be a potentially beneficial treatment for patients who exhibit prototypical levels of cognitive impairment
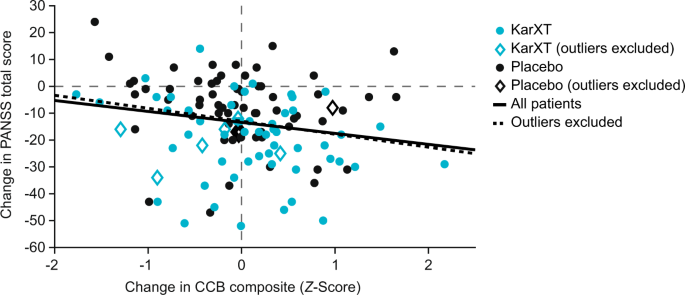

Cobenfy may be effective in improving cognitive function. We are very hopeful.
Advantage 3: Less weight gain
Patients treated with Cobenfy for one year lost an average of 2.6 kg. 65% of patients lost weight.
Cobenfy has gastrointestinal side effects. This may cause weight loss rather than weight gain.

Cobenfy causes more people to lose weight rather than gain it.
Advantage 4: Fewer extrapyramidal symptoms.
The only extrapyramidal symptom that occurred in Cobenfy more than 2% in the phase 2 trial was akathisia (3%).

Cobenfy has virtually no extrapyramidal symptoms. This is another major advantage over conventional antipsychotics.
Advantage 5: Enhanced effects as an adjunctive drug
Cobenfy is intended for use as an adjunct to existing medications. It may be effective for patients who remain with auditory hallucinations and other symptoms with the use of existing drugs alone.
It may also help treatment-resistant schizophrenia.

We do not know for sure about the use of cobenfy as an adjunctive drug, as there is not much information available yet.
However, since the mechanism of action is very different from that of existing drugs, there is a possibility that it may enhance the effect when used in combination with existing drugs.
Two Disadvantages of Cobenfy
Next, I will write about the disadvantages of taking Cobenfy.
Disadvantage 1: Need to take twice a day
Cobenfy needs to be taken twice a day. This may be troublesome for patients who are not good at managing their medications. This can make it difficult for patients to comply with their medications and can lead to relapse.
TerXT, an improved version of KarXT, and a long-acting injectable drug are said to be in development. TerXT only needs to be taken once a day.

Cobenfy needs to be taken twice a day.
Disadvantage 2: Gastrointestinal side effects
Cobenfy has been associated with gastrointestinal side effects.
In the Phase 2 study, constipation (17% occurrence) and nausea (17% occurrence) were particularly common.
Other common side effects included dry mouth (9%), dyspepsia (9%), vomiting (9%), diarrhea (2%), and decreased appetite (2%).
After one year of treatment, 15% of patients discontinued treatment due to side effects. It is not known how many of these patients discontinued due to gastrointestinal side effects.
Some patients may decide to discontinue due to gastrointestinal side effects.
It is said that most gastrointestinal side effects were mild to moderate in severity and transient.

Cobenfy is concerned about gastrointestinal side effects. It is not clear how many until they have actually been used.
To add to that, there is also a bit of concern about cardiovascular side effects, such as high blood pressure.
Conclusion
Advantages
- Cobenfy may be particularly effective for positive symptoms. Its effect on negative symptoms is unknown.
- Effects on cognitive dysfunction are also highly promising.
- Weight gain is less common. Rather, weight loss is more common.
- Extrapyramidal symptoms are said to be almost non-existent.
- Effects may be enhanced by concomitant use with existing drugs.
Disadvantages
- Cobenfy must be taken twice daily.
- Gastrointestinal side effects are a concern.
Comment
Antipsychotic drugs were invented about 70 years ago. The first antipsychotic was chlorpromazine. Since then, they have essentially treated schizophrenia by blocking dopamine.
Cobenfy indirectly suppresses dopamine activity by activating the brain’s acetylcholine system. This is the most innovative mechanism of action for treating schizophrenia since chlorpromazine.
Because it does not directly block dopamine, it has almost none of the side effects of existing drugs. It also has the potential to improve cognitive dysfunction by acting on the brain in a manner similar to acetylcholine.
I have very high expectations for this drug.
I would like to watch the feedback from patients who have taken Cobenfy in the US. I will let you know that in this blog. Please take a look at it as a reference.
Related article is here.
Innovative Schizophrenia Drugs【Summary part 1】Muscarinic Agonists
Overview of【KarXT(Cobenfy)】High efficacy, Less Weight Gain & Less EPS
[democracy id=”114″]References
- https://pubmed.ncbi.nlm.nih.gov/33626254/
- https://pubmed.ncbi.nlm.nih.gov/38104575/
- https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2818047#google_vignette
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6891890/
- https://psychiatryonline.org/doi/full/10.1176/appi.ajp.2008.06091591
- https://www.nature.com/articles/s41398-022-02254-9
