Introduction
The results of Emraclidine’s Phase 2 trials have been released. Emraclidine is an antipsychotic drug that acts on muscarinic receptors.
Two Phase 2 trials, known as the Empower-1 and Empower-2 trials, were conducted. Both trials failed to demonstrate efficacy and ended in failure.
On this blog, we have previously stated that muscarinic-based drugs generally exhibit high efficacy. However, that assumption is becoming less certain.
Other muscarinic-based drug, Cobenfy, has consistently shown high efficacy. As for Emraclidine and NBI-1117568, it is still unclear whether they will demonstrate high or low efficacy.
In this article, we will first take a look at just how low the efficacy of Emraclidine was in these trials. Since this is a discussion about a failed clinical trial, it might not be very engaging.
Afterward, we will briefly consider why the efficacy turned out to be so low.
The data disclosed from these clinical trials are summarized in the table below. In these trials, Emraclidine was administered for six weeks.
| Empower-1 | Placebo | Emraclidine 10mg | Emraclidine 30mg |
| PANSS score before medication | 98.3 | 97.6 | 97.9 |
| Mean score reduction by medication | -13.5 | -14.7 | -16.5 |
| Empower-2 | Placebo | Emraclidine 15mg | Emraclidine 30mg |
| PANSS score before medication | 97.4 | 98.0 | 97.2 |
| Mean score reduction by medication | -16.1 | -18.5 | -14.2 |
For details on PANSS (Positive and Negative Syndrome Scale) scores, please refer to the article linked below.
Positive and Negative Syndrome Scale(PANSS, Scale used to measure efficacy)
In the PANSS score, higher values indicate greater severity of schizophrenia. The efficacy of a trial drug is measured by the extent to which it can reduce the PANSS score.
“Mean score reduction” from Emraclidine Administration
First, let’s look at one measure of efficacy: “Mean score reduction.”
The table below shows the “Mean score reduction” for other antipsychotic drugs. Additionally, it includes the “Mean score reduction” observed in the Empower-1 and Empower-2 trials of Emraclidine.
Naturally, the larger the score reduction, the higher the efficacy is considered to be.
| Study drug | Dosing period | Phase | Mean score reduction |
| Brilaroxazine | 4weeks | Phase3 | -23.9 |
| Cobenfy | 5weeks | Phase3① | -21.2 |
| Cobenfy | 5weeks | Phase3② | -20.6 |
| Ulotaront | 6weeks | Phase3① | -19.6 |
| Emraclidine | 6weeks | Phase1b | -19.5 |
| Lurasidone | 6weeks | Phase② | -19.3 |
| Emraclidine 15mg | 6weeks | Phase2 (Empower-2) | -18.5 |
| NBI-1117568 | 6weeks | Phase2 | -18.2 |
| Ulotaront | 6weeks | Phase3② | -18.1 |
| Lurasidone | 6weeks | Phase3① | -17.9 |
| Cobenfy | 5weeks | Phase2 | -17.4 |
| Ulotaront | 4weeks | Phase2 | -17.2 |
| Emraclidine 30mg | 6weeks | Phase2 (Empower-1) | -16.5 |
| Emraclidine 10mg | 6weeks | Phase2 (Empower-1) | -14.7 |
| Lumateperone | 4weeks | Phase3 | -14.5 |
| Emraclidine 30mg | 6weeks | Phase2 (Empower-2) | -14.2 |
When Emraclidine was administered at 15 mg, the score reduction was -18.5 points. This indicates an average level of efficacy.
However, at other doses (10 mg and 30 mg), the score reductions could not be considered sufficient to demonstrate efficacy. The reductions were comparable to, or slightly higher than, those of lumateperone, placing them in the lower tier of efficacy.

In the Phase 2 trials of Emraclidine, the average reduction in PANSS scores was generally at a lower level. However, at least for the 15 mg dose, there was an average reduction in scores.
When evaluating efficacy based on the “Mean score reduction”, the results could be described as three losses and one draw.
“Difference from Placebo”
As a measure of antipsychotic efficacy, the “Difference from placebo” is also a helpful reference.
Whether a clinical trial is deemed a success or failure depends more directly on the “Difference from placebo” than the drug’s “Mean score reduction.”
The “Difference from placebo” can be calculated using the following formula:

For example, in the Empower-1 trial, administering 10 mg of Emraclidine reduced the score by an average of -14.7 points.
Meanwhile, the placebo reduced the score by an average of -13.5 points. Therefore, the “Difference from placebo” is -1.2.
Similarly, in the same Empower-1 trial, administering 30 mg of Emraclidine reduced the score by an average of -16.5 points.
The placebo reduced the score by -13.5 points, making the “Difference from placebo” -3.0.
In the Empower-2 trial, the “Difference from placebo” for the 15 mg dose of Emraclidine was -2.4.
For the 30 mg dose in the same trial, the “Difference from placebo” was +1.9. (A positive value means that the placebo produced a greater score reduction than Emraclidine.)
Generally, the “Difference from placebo” is further evaluated by dividing it by the Standard deviation, resulting in a metric called Effect size, which serves as a more refined measure of efficacy.

However, since neither the standard deviation nor the effect size has been disclosed in this case, we will assess efficacy based on the “Difference from placebo.”
The table below shows the “Difference from placebo” for other antipsychotic drugs, alongside the results from the Empower-1 and Empower-2 trials.
As expected, a larger “Difference from placebo” indicates greater efficacy.
| Study Drug | Phase | Difference from Placebo |
| Emraclidine | Phase1b | -12.7 |
| Cobenfy | Phase2 | -11.6 |
| Brilaroxazine | Phase3 | -10.1 |
| Cobenfy | Phase3① | -9.6 |
| Cobenfy | Phase3② | -8.4 |
| NBI-1117568 | Phase2 | -7.5 |
| Ulotaront | Phase2 | -7.5 |
| Lurasidone | Phase3② | -6.6 |
| Lurasidone | Phase3① | -4.8 |
| Lumateperone | Phase3 | -4.2 |
| Ulotaront | Phase3② | -3.8 |
| Emraclidine 30mg | Phase2 (Empower-1) | -3.0 |
| Emraclidine 15mg | Phase2 (Empower-2) | -2.4 |
| Emraclidine 10mg | Phase2 (Empower-1) | -1.2 |
| Ulotaront | Phase3① | -0.3 |
| Emraclidine 30mg | Phase2 (Empower-2) | +1.9 |
The “Difference from placebo” for Emraclidine was consistently at the lower tier. At all doses, the “Difference from placebo” was smaller than that of Lumateperone.

The “Difference from placebo” for Emraclidine was quite small. Consequently, it was concluded that “efficacy was not demonstrated,” resulting in the failure of this trial.
“Mean score reduction” for Placebo
A smaller “Difference from placebo” occurs not only when the study drug’s “Mean score reduction” is small but also when the placebo drug’s “Mean score reduction” is large.
While this may seem obvious, the formula below illustrates this relationship:

As mentioned earlier, the “Mean score reduction” for Emraclidine in this trial was small (with a record of three losses and one draw).
In addition, the “Mean score reduction” for the placebo drug in this trial was quite large.
As a result, the “Difference from placebo,” a key efficacy measure, was significantly small, as shown previously.
The table below shows the “Mean score reduction” for placebo drugs in clinical trials of other antipsychotic drugs, alongside the results for placebo drugs in Emraclidine’s Phase 2 trials.
| Study drug | Dosing period | Phase | Mean score reduction (placebo) |
| Ulotaront | 6weeks | Phase3① | -19.3 |
| Emraclidine | 6weeks | Phase2 (Empower-2) | -16.1 |
| Ulotaront | 6weeks | Phase② | -14.3 |
| Brilaroxazine | 4weeks | Phase3 | -13.8 |
| Emraclidine | 6weeks | Phase2 (Empower-1) | -13.5 |
| Lurasidone | 6weeks | Phase3① | -13.1 |
| Lurasidone | 6weeks | Phase3② | -12.7 |
| Cobenfy | 5weeks | Phase3② | -12.2 |
| Cobenfy | 5weeks | Phase3① | -11.6 |
| NBI-1117568 | 6weeks | Phase2 | -10.8 |
| Lumateperone | 4weeks | Phase3 | -10.3 |
| Ulotaront | 4weeks | Phase2 | -9.7 |
| Emraclidine | 6weeks | Phase1b | -6.77 |
| Cobenfy | 5weeks | Phase2 | -5.9 |
In the Phase 2 trials of Emraclidine, the “Mean score reduction” with placebo drugs was -16.1 and -13.5, which are considerably larger than average.

In this trial, the placebo drug’s “Mean score reduction” was remarkably large. This reduced the “Difference from placebo,” resulting in the trial’s failure.
The primary reason for the trial’s failure was the excessively large “Mean score reduction” with the placebo drug.
Summary of Efficacy Assessment
- The “Mean score reduction” with Emraclidine was small (only the 15 mg dose was average).
- The “Mean score reduction” with placebo drugs was quite large (the main reason for the trial’s failure).
- As a result, the “Difference from placebo” was small, leading to the trial’s failure.
Why Was the Efficacy Low in This Trial?
The low efficacy of Emraclidine observed in this trial could be attributed to the following four factors:
①Emraclidine enhances the sensitivity of M4 receptors rather than acting directly on them. (In other words, it is not an M4 receptor agonist but rather an M4 allosteric agonist.)
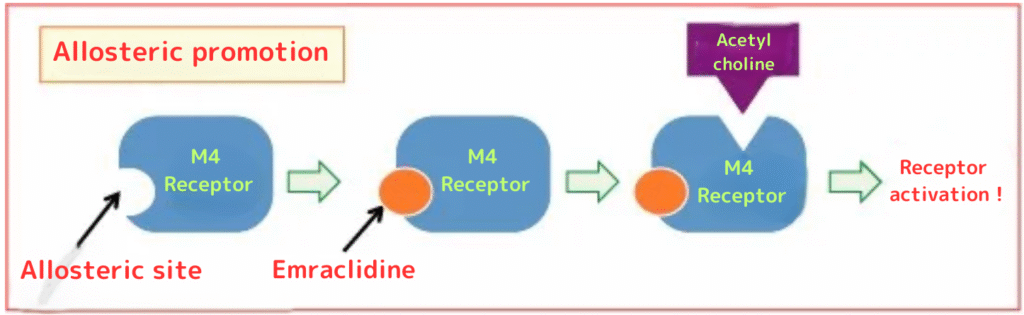
(In the picture above, Emraclidine, as an M4 allosteric agonist, binds to the allosteric site of the M4 receptor. This promotes acetylcholine binding to the M4 receptor (allosteric promotion). In the case of an M4 receptor agonist, the drug would directly bind to the same site as acetylcholine. Without directly binding, receptor activation might be insufficient.)
②Emraclidine only acts on M4 receptors and has no effect on M1 receptors. (Unlike Cobenfy, which is an M1/M4 receptor agonist, Emraclidine is solely an M4 allosteric agonist.)
③Flaws in the trial design.(The way the trial was structured may have been problematic.)
④Unknown factors.
①② Are M4 Allosteric Agonists Ineffective?
There is a possibility that factors ① and ② are responsible. Since these have been discussed in detail elsewhere, I will omit an in-depth explanation here.
In the future, results from clinical trials involving drugs like ML-007 (an M1/M4 agonist), ANAVEX3-71 (an M1 agonist), and M1 agonists or M1/M4 agonists being developed by Nexera and Neurocrine Biosciences are expected.
It will be important to closely monitor these developments.
Additionally, Addex Therapeutics is developing another M4 allosteric agonist, separate from AbbVie’s.

M4 allosteric agonists, like Emraclidine, might lack sufficient efficacy. Moving forward, we need to carefully evaluate the results of other muscarinic-based drugs to assess this further.
③ Flawed Trial Design
Another possibility is that the trial design was flawed, leading to a large reduction in average scores for the placebo group and consequently smaller efficacy metrics.
In the Phase 1b trial, only 5 facilities participated. However, the Phase 2 trial involved nearly 30 facilities, and the patient population was multinational.
Moreover, disease severity was assessed via online interviews rather than in-person evaluations, which might have made accurate assessments challenging. This could have contributed to the significant improvements observed in the placebo group.
AbbVie, the developer of Emraclidine, plans to carefully examine the data from the Phase 2 trial to determine its next steps.
There is a possibility that they may skip another Phase 2 trial and move directly to properly designed Phase 3 trials. That kind of thing is possible, in a way.
However, it’s also possible that the drug’s development might be discontinued altogether. It will be crucial to monitor the future developments of Emraclidine.

This time, it has been suggested that the trial’s execution was flawed. If conducted properly, the efficacy results might turn out to be higher.
From the patients’ perspective, we might hope for an immediate transition to Phase 3 trials and positive outcomes.
④ Unknown Causes
There is a possibility that an unknown cause led to the large reduction in scores for the placebo group, resulting in lower observed efficacy.
For example, another antipsychotic under development, Ulotaront, also showed a significant placebo improvement with a reduction of -19.3. The reasons behind this are still unclear.
Some studies suggest that when patients exhibit severe disorganized thinking, the improvement observed with placebo treatment tends to be larger. Alternatively, unknown factors may be contributing to the increased placebo response.
In recent years, the magnitude of placebo improvement has been increasing. Therefore, it would be beneficial to develop trial methodologies that can better predict and control placebo responses.
As it stands, excessive placebo effects may result in effective drugs not being approved, which would be unreasonable. Continued research into placebo responses is urgently needed.

This time, the large reduction in scores with placebo treatment might be due to factors that have yet to be understood.
Summary of Causes for Low Efficacy
- M4 allosteric agonists may lack sufficient efficacy.
- The trial design may have been flawed.
- Unknown causes.
Comment
Concerns remain about the gastrointestinal side effects of KarXT (Cobenfy). Some patients may not tolerate Cobenfy, which is why there is hope for Emraclidine. The failure of this trial is disappointing.
By the way, Cobenfy was recently launched in the U.S., and the initial feedback appears to be positive. After 1–3 weeks of use, most users report that side effects are not severe. Some have mentioned feeling more mentally clear or experiencing improved memory.
The release of Cobenfy is a positive development, and we hope that NBI-1117568 will also successfully make it to market.
While the results for Emraclidine were disappointing, muscarinic-based therapies appear to have better-than-expected effects on cognitive functions. Let’s remain optimistic and await further progress.
Related links can be found here.
【Emraclidine】Efficacy Compared to Current Drugs in Phase1b
Innovative Schizophrenia Drugs【Summary part 1】Muscarinic Agonists
Five Advantages of Taking KarXT (Cobenfy)/ A Summary Article for Beginners
[democracy id=”117″]References
- https://soseiheptares.blogspot.com/2024/11/emraclidineph2.html
- https://www.fiercebiotech.com/biotech/abbvies-9b-schizophrenia-prospect-flunks-phase-2-trials-handing-advantage-bms
- https://seekingalpha.com/article/4736064-abbvie-emraclidines-failure-eliminates-an-important-growth-driver
- https://www.psychiatrictimes.com/view/emraclidine-for-schizophrenia-fails-to-meet-primary-endpoints-in-phase-2-empower-trials
- https://pubmed.ncbi.nlm.nih.gov/30198163/
