- Introduction
- (1) Only the low-dose(20 mg) group showed high efficacy
- (2) Is the Change from Baseline(-18.2) large?
- (3) Is the “Difference from placebo”(-7.5) large?
- (4) Is the effect size of 0.61 large?
- (5) Mild side effects, but slightly sedating?
- Summary of this article
- PS: Is the placebo improvement(-10.8) large?
- Comment
- References
Introduction
The results of the Phase 2 study of NBI-1117568 are now available. I will discuss the resulting data.
The results of this clinical trial are complicated, and it is difficult to say whether the results are good or bad. I will say both positive and negative depending on the item of data.
To get straight to the point, from the patient’s point of view, and if I believe what Neurocrine is saying, the results were good.
In this article I will discuss the following five things
- I believe what Neurocrine says concerning higher efficacy at a lower dose and lower efficacy at higher doses.
- The “Change from Baseline” of -18.2 is said to be large, but it is about normal.
- The “Difference from Placebo” was 7.5. It is not very large.
- The effect size of 0.61 is large. If we look at efficacy by effect size, it would be higher than olanzapine and about the same efficacy as KarXT and Emraclidine.
- Side effects are somewhat milder; there is no concern about gastrointestinal or cardiovascular side effects as with KarXT. Possibly more sedating.
The table below contains the resulting data on effectiveness.
| Placebo (N=68) | 20mg QD (N=35) | 40mg QD (N=38) | 60mg QD (N=34) | 30mg BID (N=26) | |
| Mean Score Reduction(Change from Baseline) | -10.8 | -18.2 | -12.6 | -13.7 | -15.8 |
| Difference from Placebo | -7.5 | -1.9 | -2.9 | -5.0 | |
| Effect Size | 0.61 | 0.27 | 0.39 | 0.23 | |
| p-value | p=0.011 | P=0.282 | p=0.189 | P=0.09 | |
| PANSS Score Change | 97 →78.8 | 95 →82.4 | 96 →82.3 | 98 →82.2 |
*In this blog, “Change from Baseline” is referred to as “Mean Score Reduction”.
These data are given using a scale called PANSS (Positive and Negative Symptom Scale).
PANSS is a scale that measures the severity of schizophrenia; the higher the PANSS score, the more severe the schizophrenia.
The efficacy of a medication is examined by the extent to which the investigational drug reduces the PANSS score.
For more information on PANSS, please refer to this article.
Positive and Negative Syndrome Scale(PANSS, Scale used to measure efficacy)
(1) Only the low-dose(20 mg) group showed high efficacy
As shown in the table above, in this Phase 2 study, only the low-dose(20 mg) group showed high efficacy.
The effect sizes for the 40 mg, 60 mg, and 30 mg twice daily groups were, in order, 0.27, 0.39, and 0.23, respectively, which are low. Only the 20 mg group has a larger effect size of 0.61.
(“Effect Size” is generally said to be large at 0.8, medium at 0.5, and small at 0.2.)
I can understand if efficacy stops increasing at higher doses above a certain level, but what does it mean to say that it is decreasing?
According to Neurocrine and Nxera, it is common in the case of psychotropic drugs for the efficacy to decrease when the dosage is increased too much.
According to Nxera, a brain may react against effects of a drug in order to maintain homeostasis, resulting in an overall decrease in the drug’s effectiveness.
However, the cause is not clearly known.
Even with Risperdal and haloperidol, increasing the dosage too much can reduce efficacy.
Emraclidine, another muscarinic antipsychotic, has also been shown to decrease the efficacy of positive symptoms at higher doses. The effect sizes on positive symptoms are 0.72 for 30 mg dose and 0.41 for 40 mg dose.
Muscarinic antipsychotics also appear to be less effective at higher doses.
Neurocrine predicted that the 20 mg dose would produce the highest efficacy in the Phase 2 trial. They strongly argue that the high efficacy of the 20 mg dose can be replicated in the Phase 3 trial.
They also had the effect size of 0.7 with the 20 mg dose in the Phase 1 trial, so it may be reproducible.

According to Neurocrine, the true efficacy of NBI-1117568 is shown in the 20 mg dose. Therefore, in the following, we will consider only the data from the 20 mg dose.
(2) Is the Change from Baseline(-18.2) large?
“Mean Score Reduction” or “Effect Size”
In this section, we will consider “Change from Baseline” or, as we refer to it in this blog, “Mean Score Reduction”.
In the phase 2 trial, the “Mean Score Reduction” of NBI-1117568 at 20 mg is -18.2.
Before considering whether this number is large or not, let us consider whether we should look at “Mean Score Reduction” or “Effect Size” when predicting efficacy in clinical practice.
When predicting the level of efficacy of an investigational drug in a clinical setting, “Mean Score Reduction” can be used as a reference, but “Effect Size” can also be used as a reference.
“Effect Size” is calculated as follows. (The numbers in red are from the Phase 2 study of NBI-1117568.)
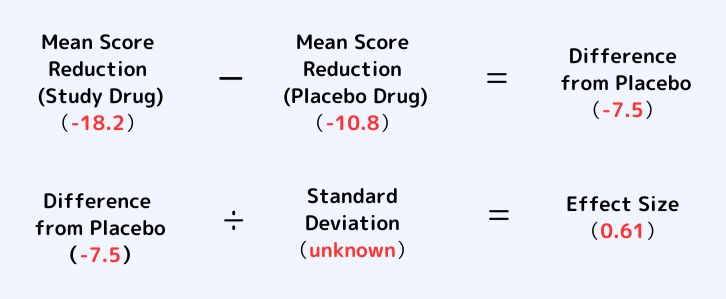
*The “Difference from Placebo” -7.5 is not an arithmetic mean, but a least squares mean value. Therefore, it is not -7.4.
*Standard Deviation indicates the degree of variability of data. The smaller the variation of the data, the more consistent and reproducible it is, and the larger the effect size.
As you can see from the formula above, “Mean Score Reduction” of a study drug is a value before a “placebo effect”(-10.8) is subtracted.
On the other hand, “Effect Size” is calculated by subtracting “Mean Score Reduction” of a placebo from “Mean Score Reduction” of a study drug, and then dividing this value by “Standard Deviation”.
Therefore, “Effect Size” is a value after a “placebo effect” (-10.8) is subtracted.
In actual treatment, a placebo effect does occur. Therefore, physicians might give more weight to “Mean Score Reduction” that includes a placebo effect.
However, some believe that subtracting a placebo effect is more indicative of the true efficacy of a study drug.
It is generally said that we need to look at both “Effect Size” and “Mean Score Reduction”.

To predict efficacy in actual treatment settings, “Effect Size” and “Mean Score Reduction” are looked at. It is generally said that both need to be looked at.
Is the “Mean Score Reduction”(-18.2) large?
Is the “Mean Score Reduction” (-18.2) for NBI-1117568 large?
As far as we know, the “Mean Score Reduction” of other antipsychotics is as shown in the table below.
| Study drug | Dosing period | Phase | Mean score reduction |
| Brilaroxazine | 4weeks | Phase3 | -23.9 |
| KarXT | 5weeks | Phase3① | -21.2 |
| KarXT | 5weeks | Phase3② | -20.6 |
| Emraclidine | 6weeks | Phase31b | -19.5 |
| Lurasidone | 6weeks | Phase3② | -19.3 |
| NBI-1117568 | 6weeks | Phase2 | -18.2 |
| Lurasidone | 6weeks | Phase3① | -17.9 |
| KarXT | 5weeks | Phase2 | -17.4 |
| Ulotaront | 4weeks | Phase2 | -17.2 |
| Lumateperone | 4weeks | Phase3 | -14.5 |
These figures are not simply comparable because of the different study designs, including the Dosing period. They are approximate.
The average of the “Mean Score Reduction” for all the study drugs in this table is -18.97. Therefore, the “Mean Score Reduction” of -18.2 for NBI-1117568 is about average.
It is greater than the -17.4 in the KarXT Phase 2 study, but less than the -21.2 and -20.6 in the two KarXT Phase 3 studies. It is also less than the -19.5 in the Phase 1b trial of Emraclidine.

Looking at “Mean Score Reduction”, one of the measures of effectiveness, the effectiveness of NBI-1117568 was normal.
(3) Is the “Difference from placebo”(-7.5) large?
Next, I will discuss the “Difference from placebo“.
“Difference from placebo” is, of course, calculated as follows. (The numbers in red are from the Phase 2 study of NBI-1117568.)

“Mean Score Reduction” and “Effect Size” mentioned earlier were looked at to predict efficacy in actual medical practice.
On the other hand, “Difference from placebo” is used to see how much better the investigational drug is than placebo for approval.
Roughly speaking, “Difference from placebo” is a value that investors, developers, regulators, etc. focus on, rather than a value that patients and doctors look at.
The “Difference from placebo” in this Phase 2 study was -7.5. Is this large?
As far as we know, the “Difference from placebo” for other antipsychotics is shown in the table below.
| Study drug | Phase | Difference from Placebo |
| Emraclidine | Phase1b | -12.7 |
| KarXT | Phase2 | -11.6 |
| Brilaroxazine | Phase3 | -10.1 |
| KarXT | Phase3① | -9.6 |
| KarXT | Phase3② | -8.4 |
| NBI-1117568 | Phase2 | -7.5 |
| Ulotaront | Phase2 | -7.5 |
| Lurasidone | Phase3② | -6.6 |
| Lurasidone | Phase3① | -4.8 |
| Lumateperone | Phase3 | -4.2 |
The average “Difference from placebo” of all the study drugs in this table is -8.3. Therefore, the “Difference from placebo” of -7.5 for NBI-1117568 is in the lower middle of the range. It is not very large.
It is considerably smaller than the -12.7 in the Phase 1b study of Emraclidine, and smaller than the -11.6, -9.6, and -8.4 in the three KarXT studies.

The “Difference from placebo” in the NBI-1117568 Phase 2 study was not very large; Neurocrine were aiming for 8 or more, but, I think, even 8 is small. I believe muscarinic agonists can do better. I would like to see more than 9 in the Phase 3 trial.
(4) Is the effect size of 0.61 large?
Next, we discuss the effect size.
In this blog, I’m most interested in effect sizes. When comparing efficacy of antipsychotic drugs, “Effect Size” is usually used most often.
“Effect Size” is “Difference from Placebo” divided by the “Standard Deviation”. (The numbers in red are from the Phase 2 study of NBI-1117568.)

“Standard Deviation” represents the degree of variation in data. The less variation, the higher the consistency and reproducibility, and the larger the effect size.
It is said that in the Phase 2 study of NBI-1117568, the variability of the data was small. In other words, the “Standard Deviation” was small.
Therefore, even though the “Difference from placebo” was not very large (-7.5), the effect size was large (0.61).
The effect sizes for other antipsychotics were calculated in one meta-analysis as shown in the table below.
(The meta-analysis combined the results of hundreds of clinical trials. The level of evidence is higher than the results of individual trials.)
| PANSS Total | Effect size | ||||
| Cloza pine | 0.89 | Sulpiride | 0.48 | Asena pine | 0.39 |
| Ami sulpride | 0.73 | Chlorpro mazine | 0.44 | Lurasi done | 0.36 |
| Olanza pine | 0.56 | Quetia pine | 0.42 | Caripra zine | 0.34 |
| Risperi done | 0.55 | Aripipra zole | 0.41 | Iloperi done | 0.33 |
| Paliperi done | 0.49 | Ziprasi done | 0.41 | Brexpipra zole | 0.26 |
| Haloperi dol | 0.47 | Sertin dole | 0.40 |
The effect size of 0.61 in the Phase 2 study of NBI-1117568 is greater than olanzapine‘s 0.56. NBI-1117568 may be more effective than olanzapine. Olanzapine itself is also a very good antipsychotic.
But it may be less effective than Clozapine or Amisulpride.
For KarXT, the Phase 2 study produced an effect size of 0.75, and the two Phase 3 studies produced effect sizes of 0.61 and 0.60.
Emraclidine has an effect size of 0.68 in a Phase 1b study.
NBI-1117568 had an effect size of 0.7 in the Phase 1 study (20 mg dose group).
So, I believe that NBI-1117568 may be as effective as other muscarinic antipsychotics (KarXT, Emraclidine).

The effect size of NBI-1117568 is greater than olanzapine; it may be as effective as KarXT or Emraclidine. As a result of the small variation in the data, the efficacy of NBI-1117568 is high when viewed in terms of effect size.
(5) Mild side effects, but slightly sedating?
Next, I will discuss the side effects of NBI-1117568.
The side effects that occurred in more than 5% of patients in this Phase 2 study were somnolence, dizziness, nausea, and constipation.
I will compare this to the side effects that occurred in more than 5% of patients with KarXT and Emraclidine, the other muscarinic antipsychotics.
First, let’s look only at gastrointestinal side effects, which are common with KarXT.
| NBI-1117568 | KarXT | Emraclidine | |
| Nausea | 5% | 17% | 7% |
| Constipation | 5% | 17% | (below 5%) |
| Indigestion | (below 5%) | 9% | (below 5%) |
| vomiting | (below 5%) | 9% | (below 5%) |
| Dry mouth | (below 5%) | 9% | 6% |
The table shows that gastrointestinal side effects are prominent with KarXT. Emraclidine and NBI-1117568 do not seem to have particularly high gastrointestinal side effects.
Next, let’s look at the most common side effects for Emraclidine.
| NBI-1117568 | KarXT | Emraclidine | |
| Headache | (2.5%) | 7% | 28% |
| Back pain | (below 5%) | (below 5%) | 6% |
The most common side effects of Emraclidine are headache and back pain. Headache occurs 28% of the time.
Next, let’s look at the most common side effects for NBI-1117568.
| NBI-1117568 | KarXT | Emraclidine | |
| Somnolence | 12.5% | 6% | 6% |
| Dizziness | 12.5% | (below 5%) | 6% |
Somnolence and dizziness were particularly common in NBI-1117568. It occurred about twice as often as the others.

Somnolence is a concern with NBI-1117568. But somnolence may be good for insomniac or agitated patients.
Summary of this article
- The true efficacy of NBI-1117568 appears in the 20 mg dose.
- The “Mean Score Reduction” of -18.2 is about normal. When looking at efficacy in terms of “Mean Score Reduction”, efficacy is about normal.
- The “Difference from placebo” of -7.5 is not very large. I would like to see more in the Phase 3 trial.
- Effect size of 0.61 is large. Possibly more effective than olanzapine, possibly as effective as KarXT or Emraclidine. The effect size was large because of the small variability of the data.
- Side effects are small. But somnolence is a common side effect. May be good for insomniac patients.
PS: Is the placebo improvement(-10.8) large?
As a follow-up, we also consider whether the placebo “Mean Score Reduction” was larger in this NBI-1117568 Phase 2 study.
The right-hand column of the table below shows the “Mean Score Reduction” with placebo administration.
| Study drug | Dosing period | Phase | Mean score reduction (Study drug) | Mean score reduction (placebo) |
| Brilaroxazine | 4weeks | Phase3 | -23.9 | -13.8 |
| Lurasidone | 6weeks | Phase3① | -17.9 | -13.1 |
| Lurasidone | 6weeks | Phase3② | -19.3 | -12.7 |
| KarXT | 5weeks | Phase3② | -20.6 | -12.2 |
| KarXT | 5weeks | Phase3① | -21.2 | -11.6 |
| NBI-1117568 | 6weeks | Phase2 | -18.2 | -10.8 |
| Lumateperone | 4weeks | Phase3 | -14.5 | -10.3 |
| Ulotaront | 4weeks | Phase2 | -17.2 | -9.7 |
| Emraclidine | 6weeks | Phase1b | -19.5 | -6.77 |
| KarXT | 5weeks | Phase2 | -17.4 | -5.9 |
The average of the “Mean Score Reduction” for all placebo drugs in this table is -10.69. Therefore, the placebo “Mean Score Reduction” of -10.8 in the NBI-1117568 Phase 2 study is about normal.
I have heard that “Mean Score Reduction” for placebo is generally around -10 points recently. Therefore, -10.8 is about normal.
By the way, looking at the table above, the placebo improvement in the KarXT Phase 2 trial was -5.9, which is quite small.
Because of the small -5.9, the “Difference from Placebo” is also larger, resulting in a larger effect size of 0.75.
Emraclidine also has a small placebo improvement of -6.77 in the Phase 1b trial. Therefore, the effect size was also larger at 0.68.
Phase 1 trials are likely to have large effect sizes. In the Phase 2 trial of Emraclidine, the placebo improvement will be much larger and the effect size will be lower. I expect it to be around 0.6.
In that case, NBI-1117568 and Emraclidine would have same degree of efficacy. In my prediction, Emraclidine and NBI-1117568 have same degree of efficacy.

Effect sizes may be greater or less, depending on the extent of improvement of a placebo.
In the Phase 2 study of NBI-1117568, the “Mean Score Reduction” for placebo was about normal. The placebo improvement range was neither particularly large nor small.
Comment
Although the results of the Phase 2 trial were mixed, NBI-1117568 is still promising for patients. I hope the results of the Phase 3 trial will be good.
In the U.S., NBI-1117568 is expected to be launched in 2029. That is still a long way off.
I heard that the Phase 3 trial can start in the first half of 2025, so I expect the launch date to be earlier.
What we have discovered is that NBI-1117568 has more somnolence side effects than KarXT and Emraclidine. Perhaps it is a more sedative drug.
Some patients with schizophrenia may prefer to be sedated. Some patients are insomniacs, so taking NBI-1117568 at night might be a good idea.
When deciding which to use among several muscarinic antipsychotics, one might choose NBI-1117568 if sedation is needed.
I will have to look closely to see if NBI-1117568 really has stronger sedative effects.
NBI-1117568 is also expected to be effective in improving cognitive dysfunction, have almost no extrapyramidal symptoms, and have almost no weight gain.
I would like to see the drug seek an indication as an adjunct to existing medications, like KarXT. This is desired for patients with treatment-resistant schizophrenia.
Related article.
Innovative Schizophrenia Drugs【Summary part 1】Muscarinic Agonists
References
- https://note.com/saburau/n/n4ea47ce590bc
- https://www.nikkei.com/nkd/disclosure/tdnr/20240828577868/
- https://markets.businessinsider.com/news/stocks/buy-rating-affirmed-for-neurocrine-biosciences-amid-promising-drug-candidate-results-1033730263
- https://www.biopharmadive.com/news/neurocrine-schizophrenia-results-data-muscarinic-bristol-abbvie/725499/
- https://www.biospace.com/drug-development/neurocrine-shares-drop-20-despite-meeting-primary-endpoint-in-mid-stage-schizophrenia-trial
- https://soseiheptares.blogspot.com/2024/08/m4p2.html
- https://soseiheptares.blogspot.com/2024/09/35.html?m=1
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6891890/
