Introduction
I have written several times that I am most excited about muscarinic agonists such as KarXT, Emraclidine, and NBI-1117568.
However, I am equally excited about Ulotaront, a TAAR1 agonist (trace amine-associated receptor 1 agonist).
Ulotaront is expected to be launched in Japan in 2026. When it becomes available, I hope to use it myself. In the U.S., it is targeted for launch in 2024.
I am currently taking olanzapine. Even if I can’t switch to Ulotaront completely, I may be able to use a small additional dose of Ulotaront.
That alone could have a number of benefits, including reduced side effects.
For example, adding a small amount of Ulotaront to an existing drug may prevent weight gain and brain atrophy due to improved metabolism.
It may also reduce extrapyramidal symptoms, improve cognitive dysfunction, and potentiate effects on positive and negative symptoms.
All good things happen, but since it has only been confirmed in animal studies, we do not know if it has the same effect in humans. I would like to make one article about it. (Might not be able to make it.)
Ulotaront also modulates serotonin and glutamate, so it may be possible to expand its use to other mental disorders in addition to schizophrenia.
It also has antidepressant and anxiolytic effects. In the U.S., it is said that clinical trials for adjunctive therapy of major depressive disorder are already underway.
In Part1, we will discuss the side effects and development status and TAAR1 agonist. The efficacy of Ulotaront will be discussed in Part2. Here is the link.
New Antipsychotics 3: Ulotaront Part2 (Phase2 efficacy data)
Part1 is slightly more difficult, so part2 is more recommended & important.
TAAR1 agonist
Ulotaront acts as a TAAR1 agonist (Trace Amine-Associated Receptor 1 agonist).
It also act strongly as a 5HT1A receptor agonist.
Six types of TAARs, TAAR1 to TAAR6, have been found in the human body.
The Trace Amine–Associated Receptors (TAARs) were first called simply Trace Amine Receptors (TARs). This was because all receptors in this family were thought to respond to Trace Amines (TA).
However, further analysis revealed that not all TARs (Trace Amine Receptors) respond to TAs (Trace Amines), and the name was changed to TAAR (Trace Amine–Associated Receptor).
Ulotaront acts on type 1 (TAAR1).
TA (Trace Amines) that act on TAARs include tyramine, phenylethylamine (PEA), octopamine, and tryptamine.
These trace amines are produced by enzymatic conversion of dopamine and serotonin precursors. Some are also produced by conversion from dopamine itself.
These trace amines (TAs) can also be produced in brain, unlike drugs such as Ulotaront. However, like Ulotaront, they also seem to act as agonists of TAARs and stimulate TAARs.
For example, tyramine stimulates TAAR1 in the midbrain (part of the brainstem) to “suppress” activity in that region.
TAAR1 agonists such as Ulotaront, like tyramine, also stimulate TAAR1 and work to inhibit dopamine activity in the midbrain and striatum. Thereby suppressing positive symptoms.
TAAR1 agonists also increase the excitability of glutamatergic neurons in the prefrontal cortex and modulate the release of serotonin. This improves negative symptoms and cognitive dysfunction.
In case you are wondering, the locations of the midbrain, striatum, and (pre)frontal cortex are shown in the figure below.
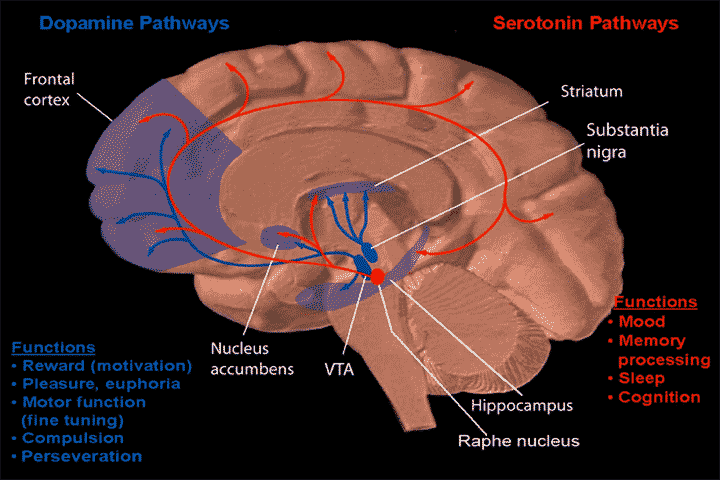
The midbrain is located in the upper part of the brainstem. The ventral tegmental area(VTA), the Raphe nucleus, and Substantia nigra are part of the midbrain. The Striatum is located above the midbrain. The Frontal cortex is located around the forehead.
In the figure, the blue lines are dopamine pathways and the red lines are serotonin pathways.
The latest findings suggest that positive symptoms are caused by an overactivity of the dopamine pathway that extends from the substantia nigra (midbrain) to the striatum. TAAR1 agonists suppress this overactivity.
Negative symptoms and cognitive dysfunction are thought to be caused by a hypoactivity of the dopamine pathway that extends from the VTA (midbrain) to the prefrontal cortex.
In addition to dopamine, TAAR1 agonists modulate glutamate and serotonin in the prefrontal cortex, thereby improving negative symptoms and cognitive dysfunction.
Development Status
Ulotaront (SEP-363856) is being developed in the U.S. by Sunovion, which announced the start of a Phase 3 trial in September 2019.
On September 30, 2021, Sunovion and Dainippon Sumitomo Pharma signed a license agreement with Otsuka Pharmaceutical for the co-development and commercialization of four new drugs in development.
Ulotaront (SEP-363856) is included among the new drugs in development.
As of May 13, 2022, it is apparently in Phase 3 trials in the US, China and Japan.
In the U.S., it is targeted for launch in 2024. In Japan, it is expected to be launched in 2026.
Side Effects
In the Phase 2 study, there were virtually no side effects derived from D2 blockade.
Extrapyramidal symptoms occurred in 3.3% of patients on Ulotaront and 3.2% on placebo.
Weight gain was small, averaging 0.3 kg gain.
The side effects that occurred in more than 2% of patients in the 6-month long-term study were as follows.
| Side effects | Ulotaront |
| Schizophrenia | 12.2% |
| Headache | 11.5% |
| Insomnia | 8.3% |
| Anxiety | 5.1% |
| Somnolence | 4.5% |
| Nasopharyngitis | 4.5% |
| Nausea | 3.8% |
| Irritability | 3.2% |
| Weight decreased | 3.2% |
| Influenza | 3.2% |
| Prolactin increased | 2.6% |
| Extrapyramidal adverse events | 3.2% |
Since insomnia was present in 8.3% and somnolence in 4.5%, it may be less sedating and more stimulating than the other drugs.
This might be so since Ulotaront is also a 5HT1A receptor agonist and is also being considered for adjunctive therapy for depression.
In the 6-month long-term study, 56.4% of patients experienced at least one side effect; 5.1% of patients experienced side effects that were considered severe.
Low side effects may be a major advantage of using Ulotaront.
Comment
Ulotaront is probably the closest antipsychotic to launch in Japan. So I am actually more excited about it now than KarXT.
According to the literature referenced in this article, it appears that TAAR1 agonists would be drugs related to the glutamate hypothesis (NMDA receptor hypothesis) as well as the dopamine hypothesis.
In this sense, they may be a more fundamental treatment than D2 blockers that follow only the dopamine hypothesis. Research studies are currently underway to see if this is really the case.
However, there is also a TAAR1 partial agonist called Ralmitaront, which failed to show efficacy in Phase 2. Currently, it is being tested for efficacy on negative symptoms only.
Ulotaront might also fail in Phase 3 if short-term efficacy is the only factor. (I will write about the efficacy in Part2.)
Ulotaront is a unique drug, and I think it has various potentials that cannot be measured in the current clinical trials. So anyway, I hope that Phase 3 will yield results that will lead to marketing approval.
I will write about the effectiveness of Ulotaront in part2. Here is the link.
New Antipsychotics 3: Ulotaront Part2 (Phase2 efficacy data)
Results of Phase 3 trials are now available.
【Ulotaront】U.S. Phase 3 Studies Failed. What’s Next? / Clinical Trial Data
Additional information.
Innovative Schizophrenia Drugs【Summary part 2】Ulotaront, etc.
[democracy id=”72″]